Oil Analysis
In order to extract useful information from the gas production mechanisms explained in the previous section, the dissolved gases in the oil have to be extracted and quantified. As it might be expected, the results of the oil analysis are critical to the correct interpretation and diagnosis of the condition of a particular transformer. It is important that the results available for analysis are representative of the oil inside the transformer and as such, it is expected that they are repeatable and reproducible.
These terms, repeatable and reproducible, have specific meanings in the context of oil analysis. When results are repeatable, it means that a particular laboratory or analyst will produce the same results if the same sample is repeatedly analysed. On the other hand, results are reproducible when two different laboratories or analyst are capable of producing the same results on a given sample.
In practice, achieving perfect reproducibility and repeatability, in other words, zero variation between tests or testing entities, is not practical. Understanding the capacity of the oil laboratory to produce repeatable and reproducible results is important for the end user and ultimately the interpreter of these results.
While each laboratory has to be evaluated by its potential clients, in general, laboratories ensure good quality repeatable and reproducible results by:
- Adhering to well-known and reliable test methodologies,
- Regularly verifying the correct calibration of instruments,
- Obtaining and maintaining accreditation by a recognised assessment authority, and
- Participating in international round-robin tests.
The specific test methodology used by a particular laboratory, typically depends on the country where the laboratory is located. The majority of modern laboratories around the world analysing transformer oils use the Gas Chromatography (GC) technique. Two of the most well-known standards that prescribe how this technique should be applied are:
- ASTM D3612-02(2009) – Standard Test Method for Analysis of Gases Dissolved in Electrical Insulating Oil by Gas Chromatography (11)
- IEC 60567 ED. 4.0 – Oil-filled electrical equipment – Sampling of gases and analysis of free and dissolved gases – Guidance (12).
Gas Chromatography
In general, Chromatography is a technique used to separate a mixture of compounds into its constituent parts. It was originally developed in Russia by the botanist Mikhail S. Tsvet in 1900. While researching plant pigments, Tsvet filled a liquid-adsorption column filled with a calcium carbonate as adsorbent. Then he used a mix of ethanol as eluent. As the plant pigments, such as chlorophylls and carotenoids, separated in the column they formed bands of various colours in the column. This separation of the original mix into coloured bands gave the technique its name: chromatography from the Greek chroma (colour) and graphein (to write).
More than a hundred years later, the technique we use to analyse dissolved gases in transformer oil is, in principle, based on Tsvet’s original work. The technique is called Gas Chromatography and it is performed with an instrument conveniently named Gas Chromatograph (GC).
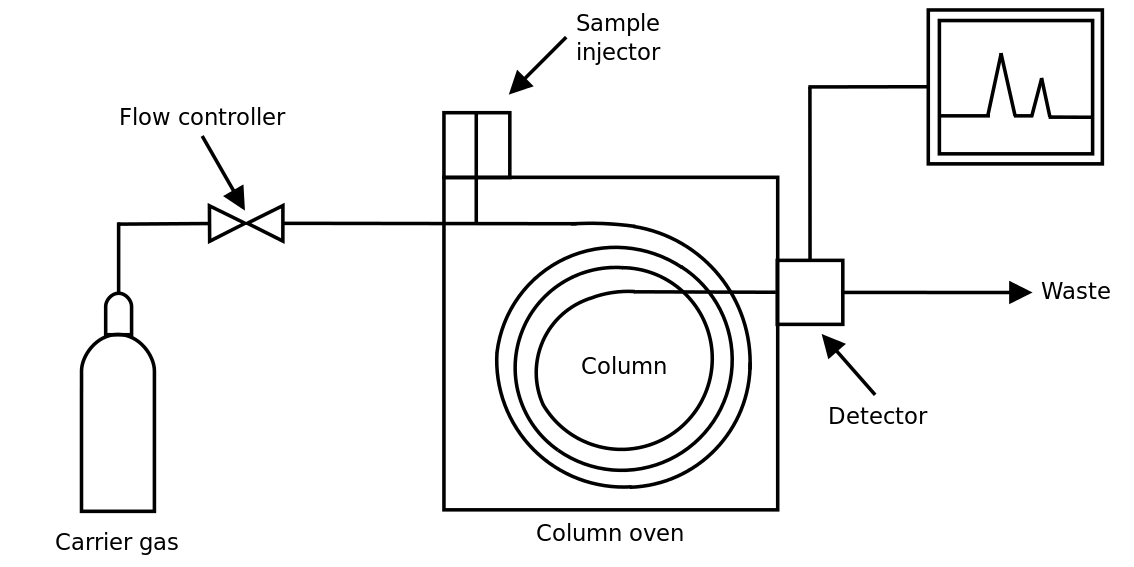
As shown in the figure below, in the case of Gas Chromatography, a gas sample of unknown composition is injected into the instrument which contains a capillary column filled with a liquid or a polymer which acts as the stationary phase. A carrier gas, typically an inert gas such as helium or nitrogen, is used as the mobile phase. As the mobile phase moves through the stationary phase in the column, the various compounds within the sample interact with the stationary phase causing them to separate and move at different speeds through the column. Each compound would therefore take a different amount of time to travel through the column which is known as the retention time. As the various compounds arrive to the end of the column, a detector or detectors will generate a signal that is recorded on a computer.
The retention time then relates to each individual compound while the area under the curve of each signal is proportional to the amount of that compound in the original mixture.
In order to be able to identify each of the compounds, the laboratory calibrates the GC by running a known mixture of the gases. This provides the laboratory with the necessary data (i.e. retention times and areas) that enable the identification of compounds and calculation of their quantity in a sample with a high level of accuracy.
Simplified representation of how a Gas Chromatograph works